Case 1
Brief HPI:
A 66-year-old male with a history of hypertension, diabetes, hyperlipidemia and prior stroke presents with acute-onset right-sided numbness. Examination demonstrates decreased sensation to light touch and pinprick in right upper- and-lower extremities as well as right face. Strength, cranial nerve and cerebellar testing is normal.
MRI Brain:
Small focus of restricted diffusion within the left insular subcortical white matter consistent with acute lacunar infarct.1
Discussion:
Infarction of the ventral posterolateral nucleus of the thalamus disrupts relays from the medial lemniscus and spinatholamic tracts extending to the cortex.
Case 2
Brief HPI:
A 34-year-old female with no significant medical history presents with headache and neck pain. Examination is notable for decreased strength with decreased sensation to pinprick (and preserved light touch) in bilateral upper extremities.
MRI C-Spine:
Chiari I malformation with associated cervical syringomyelia (79mm length, 11mm anteroposterior diameter) extending from C3 to T1.2
Discussion:
The central cord lesion affects the adjacent, medial fibers of the corticospinal tract resulting in upper extremity weakness. Disruption of crossing spinothalamic tract fibers results in diminished pain and temperature sensation at the level of the lesion.
Anatomy3,4
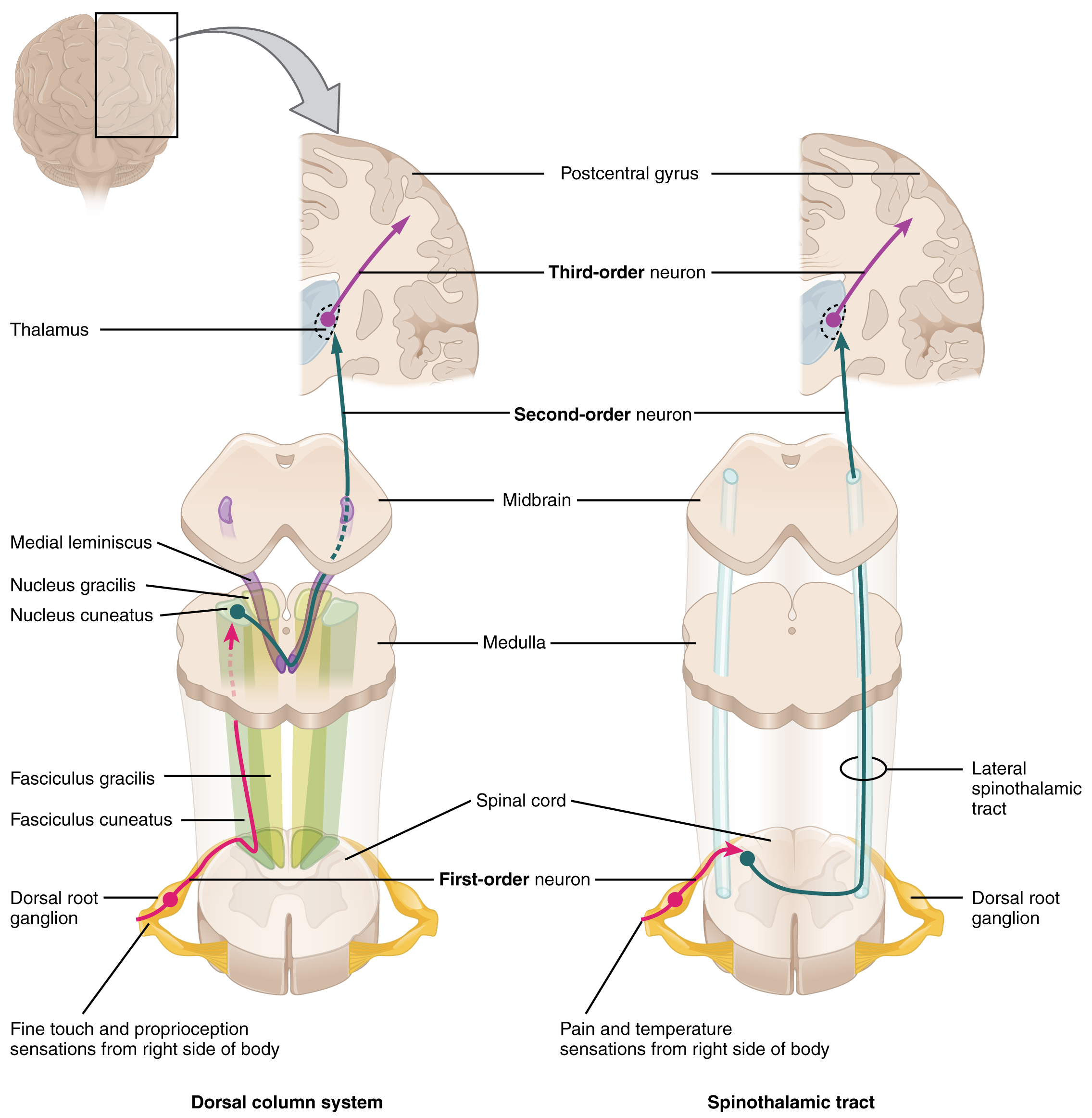
Sensory information is gathered from specialized receptors in skin and soft-tissues which detect a variety of stimuli including temperature, pressure, vibration, and pain. This is subsequently transmitted through peripheral nerves which in certain regions coalesce into larger bundles before entering the spinal cord through the dorsal nerve root. Upon entering the spinal cord, sensory information is divided into two tracts:
- Spinothalamic: Small, poorly-myelinated fibers carrying pain, temperature and touch stimuli synapse with second-order neurons over several levels in the ipsilateral dorsal horn crossing to the contralateral side in the anterior commissure anterior to the central canal. Touch information ascends in the anterior spinothalamic tract while pain and temperature information ascends in the lateral spinothalamic tract.
- Dorsal column: Large, myelinated fibers carrying vibration and proprioception information ascend in the ipsilateral posteromedial spinal cord. Fibers from the thoracic and lumbar region occupy the more medial (gracile) column, while fibers from the cervical region occupy the more lateral (cuneate) column. These fibers synapse in their respective nuclei in the medulla before crossing to the contralateral medial lemniscus.
Both tracts proceed to the ventral posterolateral (VPL) nucleus of the thalamus, through the internal capsule before terminating in the somatotopically-arranged primary somatosensory cortex in the parietal lobe.
Understanding the neuroanatomy supports a systematic approach to the evaluation of sensory disturbances. It is important to note that the transmission of light touch sensation involves both pathways and offers less localizing value when compared to specific assessment of proprioception or pain detection. Sensory disturbances are often accompanied by motor abnormalities which can further aid with localization. Other key distinguishing features include acuity of onset wherein abrupt presentations may suggest ischemia or infarction, compared to more indolent processes with broader differentials (including compressive mass lesions, demyelination, or autoimmune disease).
An Algorithm for the Evaluation of Sensory Disturbances5
References
- Case courtesy of Dr Bruno Di Muzio, Radiopaedia.org, rID: 45066
- Case courtesy of Dr Bahman Rasuli, Radiopaedia.org, rID: 65655
- Aminoff MJ. Numbness, Tingling, and Sensory Loss. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e New York, NY: McGraw-Hill; 2014. http://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79724797. Accessed February 07, 2020.
- Sensory Disorders. In: Simon RP, Aminoff MJ, Greenberg DA. eds. Clinical Neurology, 10e New York, NY: McGraw-Hill; . http://accessmedicine.mhmedical.com/content.aspx?bookid=2274§ionid=176234164. Accessed February 07, 2020.
- Berkowitz AL. Numbness: A Localization-Based Approach. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB. eds. Principles and Practice of Hospital Medicine, 2e New York, NY: McGraw-Hill; . http://accessmedicine.mhmedical.com/content.aspx?bookid=1872§ionid=146977205. Accessed February 07, 2020.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)