HPI:
A 62 year-old male with a history of hepatitis C cirrhosis complicated by hepatocellular carcinoma s/p radiofrequency ablation presenting after referral from hepatology clinic for hyponatremia. One week ago, the patient developed abdominal distension and shortness of breath that resolved after large-volume paracentesis and was started on furosemide 40mg p.o. daily and aldactone 100mg p.o. daily. After initiating diuretics, the patient noted worsening lower extremity edema, and increased thirst/fluid intake.
He reports two days of fatigue and intermittent confusion supported by family members who reported slowed speech. He otherwise denies abdominal pain, distension, nausea/vomiting, diarrhea/constipation, chest pain or shortness of breath. In the ED, the patient received 1L NS bolus.
PMH:
- Hepatitis C cirrhosis c/b HCC s/p RFA
- Rheumatoid arthritis
PSH:
- None
Family History:
- Non-contributory.
Social History:
- Lives with partner, denies current or prior t/e/d abuse
- HepC contracted from blood transfusions
Medications:
- Furosemide 40mg p.o. daily
- Spironolactone 100mg p.o. daily
- Rifaximin 550mg p.o. b.i.d.
Allergies:
- NKDA
Physical Exam:
| VS: | T | 98.2 | HR | 80 | RR | 14 | BP | 95/70 | O2 | 98% RA |
| Gen: | Elderly male in no acute distress, alert and answering questions appropriately. | |||||||||
| HEENT: | NC/AT, PERRL, EOMI, faint scleral icterus, MMM. | |||||||||
| CV: | RRR, normal S1/S2, no murmurs. JVP 8cm. | |||||||||
| Lungs: | Faint basilar crackles on bilateral lung bases. | |||||||||
| Abd: | Normoactive bowel sounds, mildly distended but non-tender, without rebound/guarding. | |||||||||
| Ext: | 2+ pitting edema in lower extremities to knees bilaterally. 2+ peripheral pulses, warm and well perfused. | |||||||||
| Neuro: | AAOx3. CN II-XII intact. No asterixis. Normal gait. Normal FTN/RAM. | |||||||||
Labs/Studies:
- BMP (admission): 112/5.6/88/22/28/1.1/97
- BMP (+10h): 118/5.4/93/23/26/1.0/133
- sOsm: 264
- Urine: Na <20, K 26, Osm 453
- BNP: 40
- AST/ALT/AP/TB/Alb: 74/57/91/2.4/2.2
Assessment/Plan:
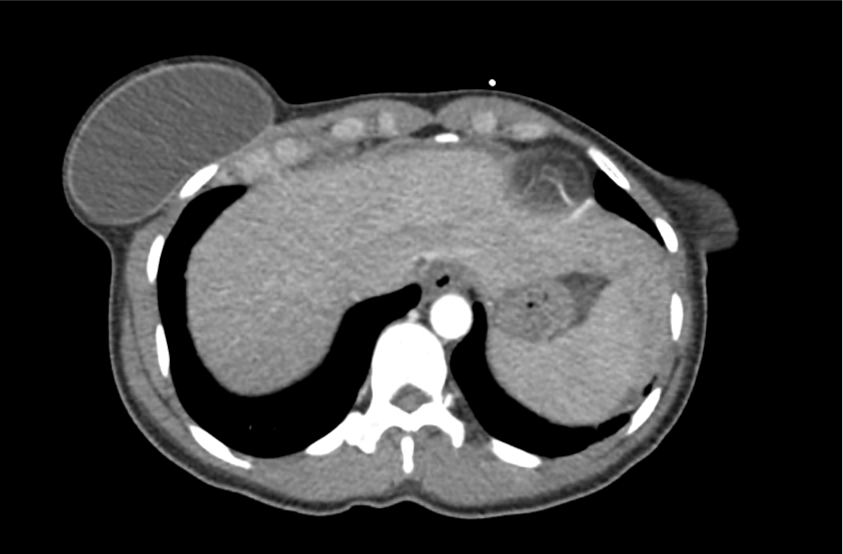
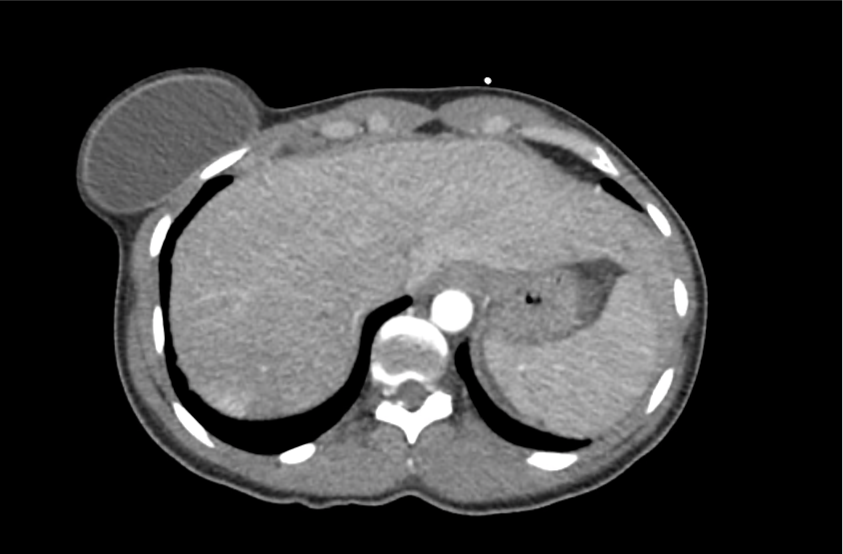
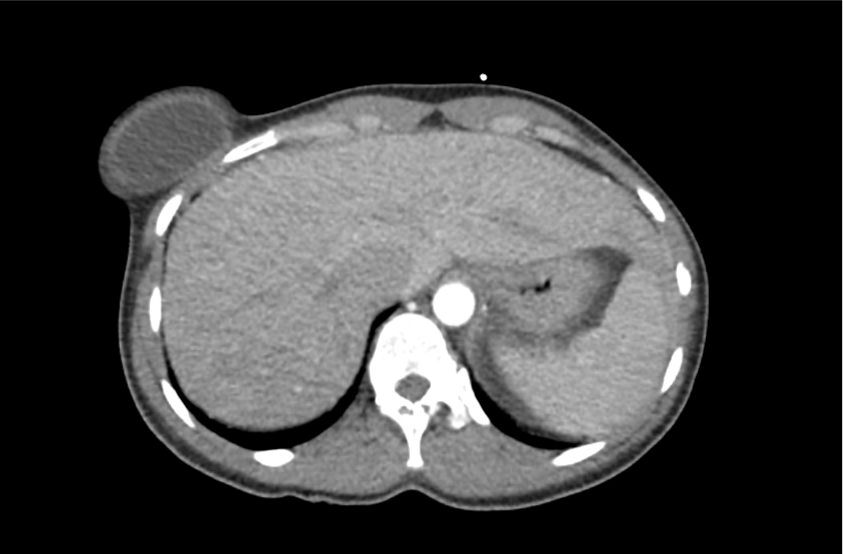
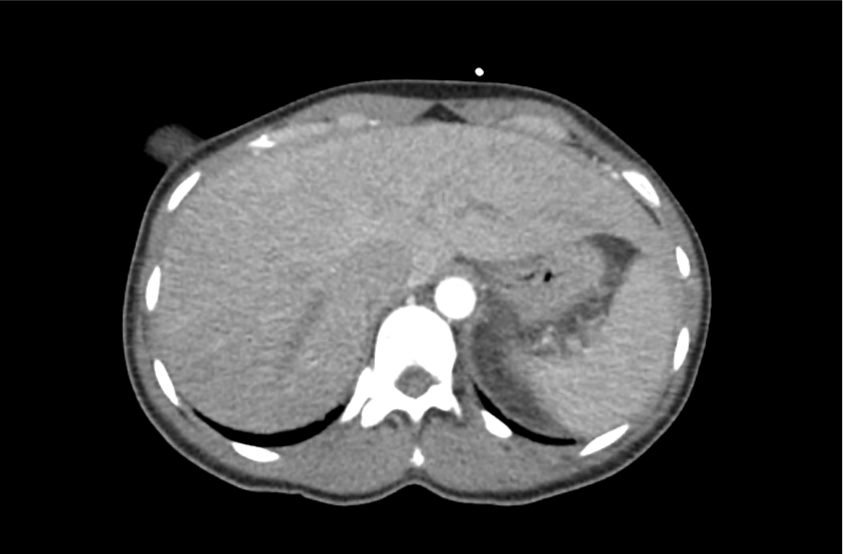
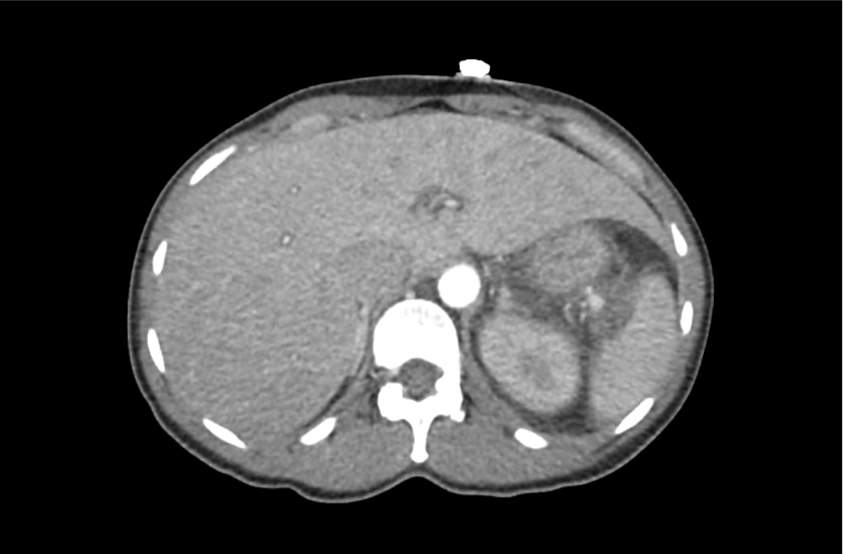
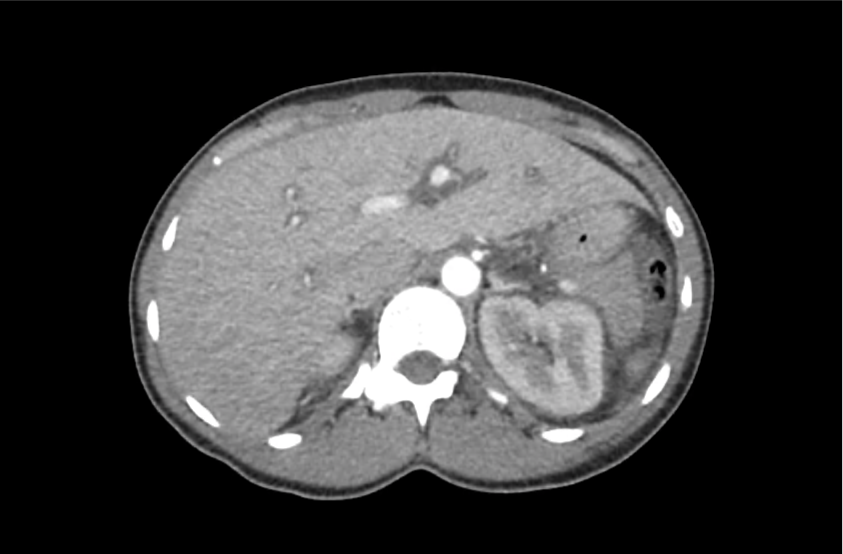
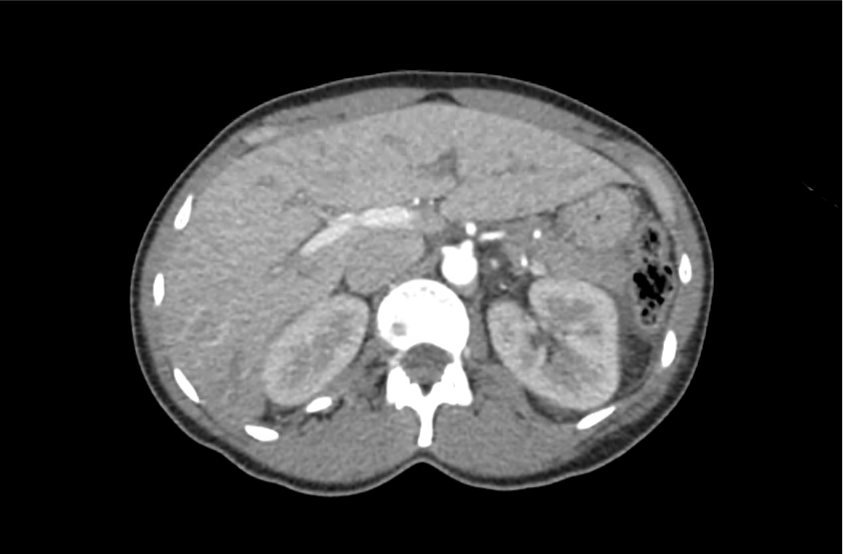
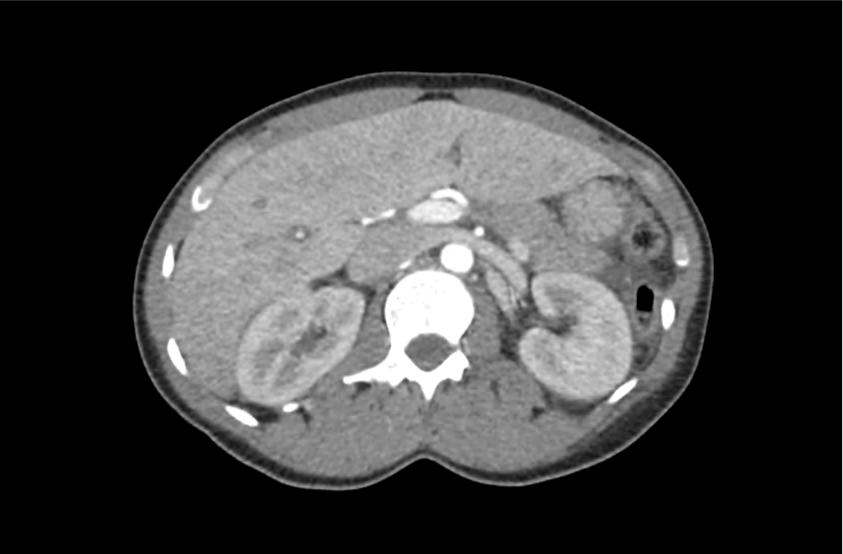
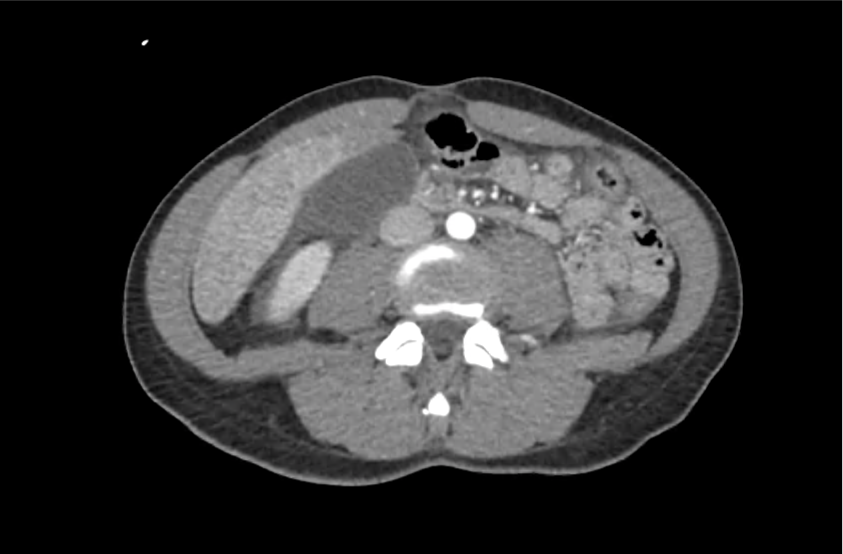
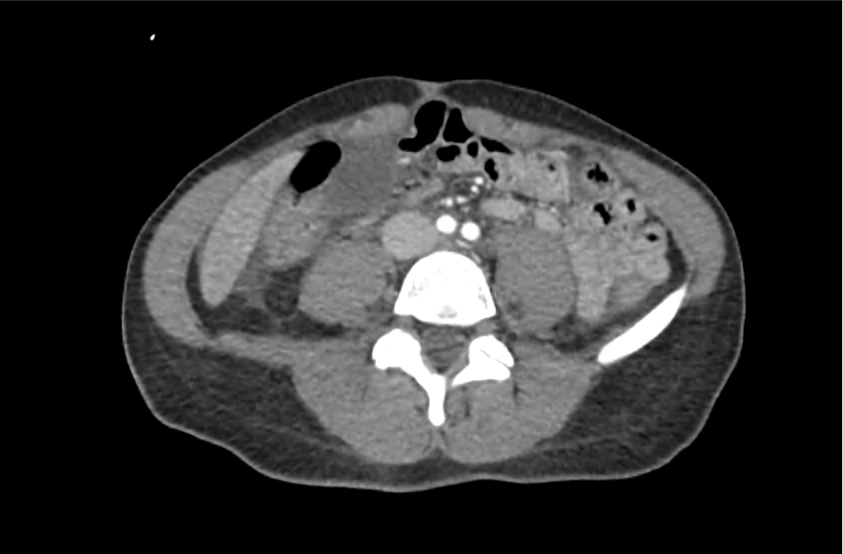
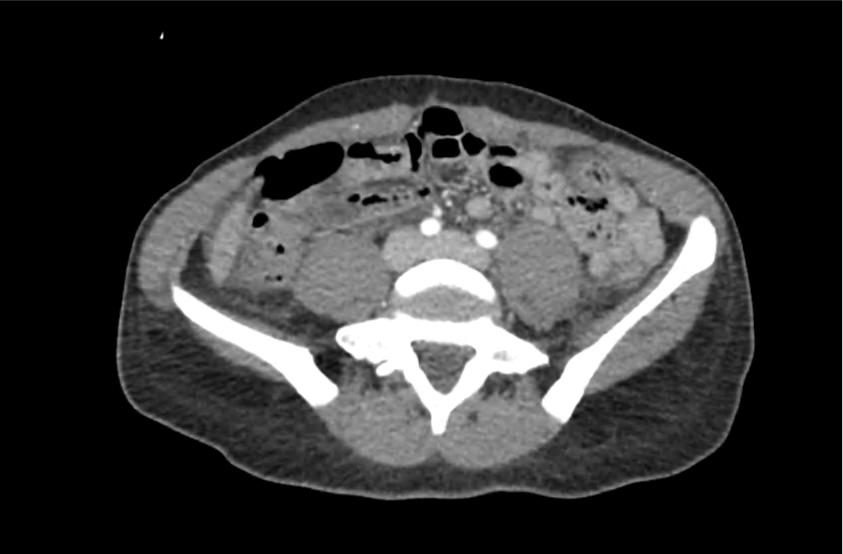
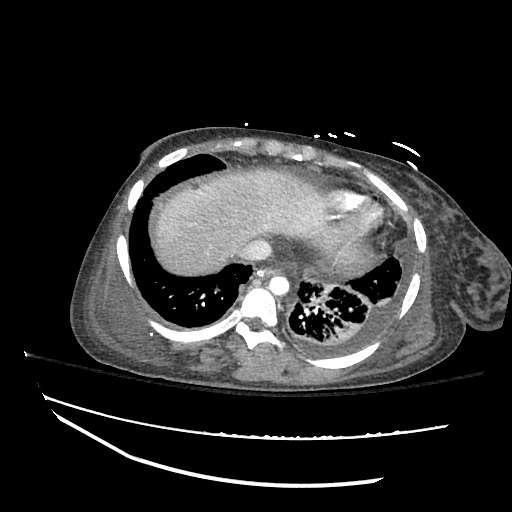
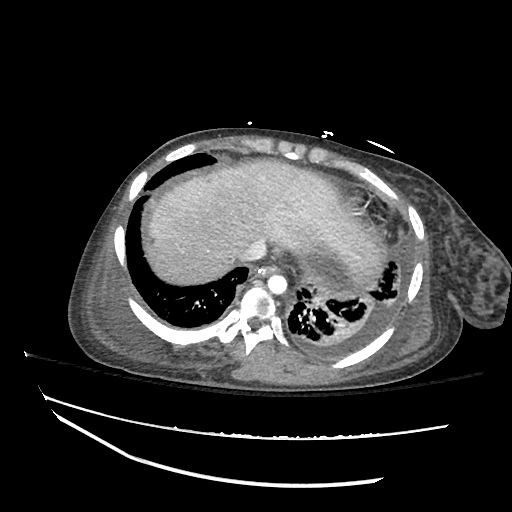
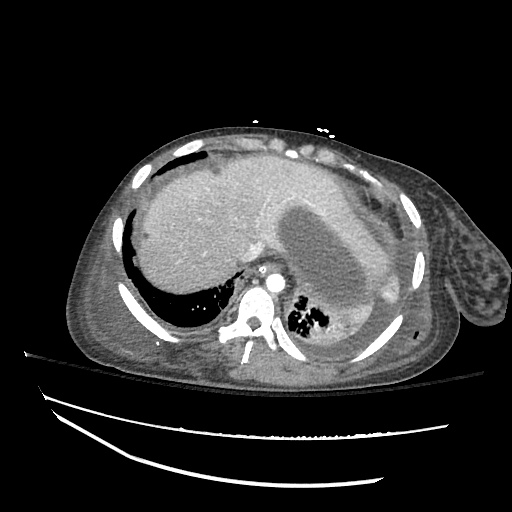
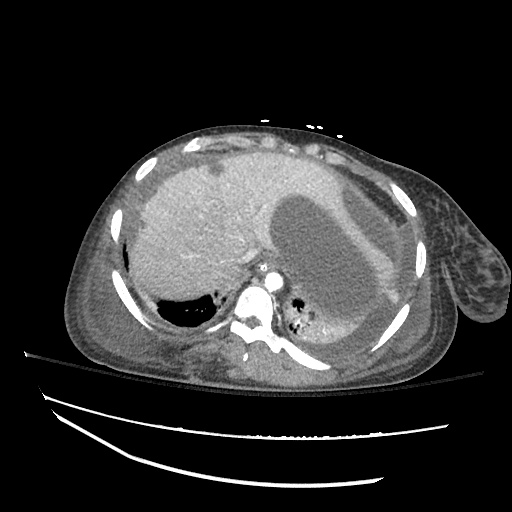
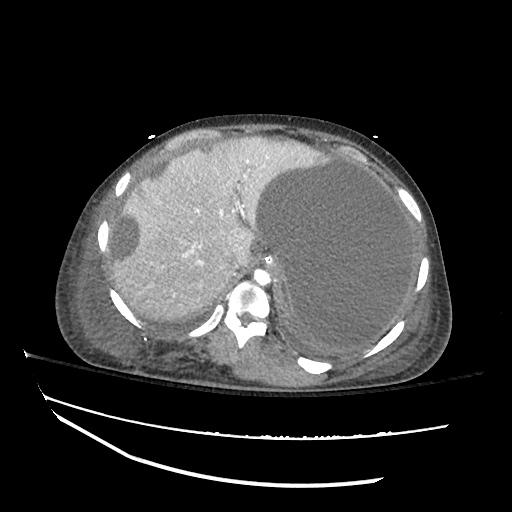
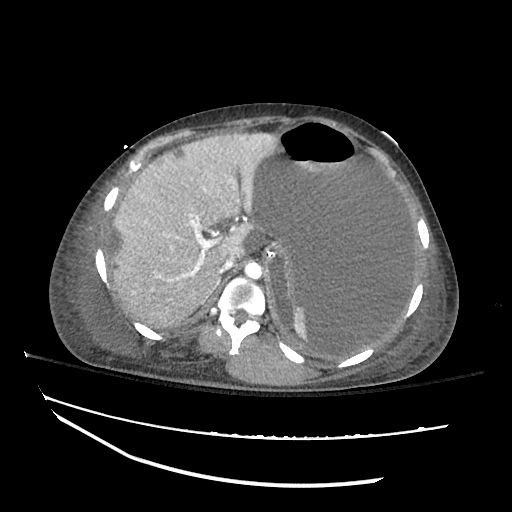
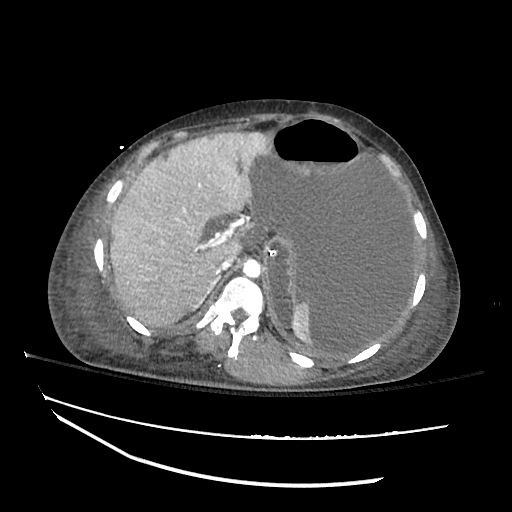
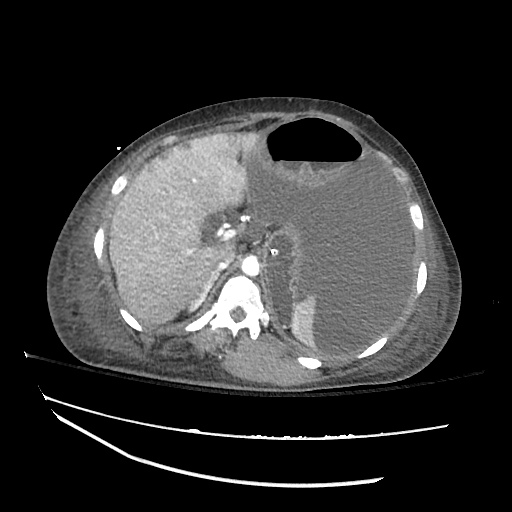
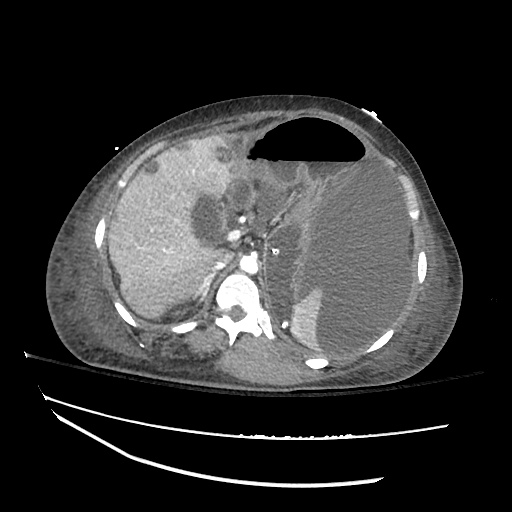
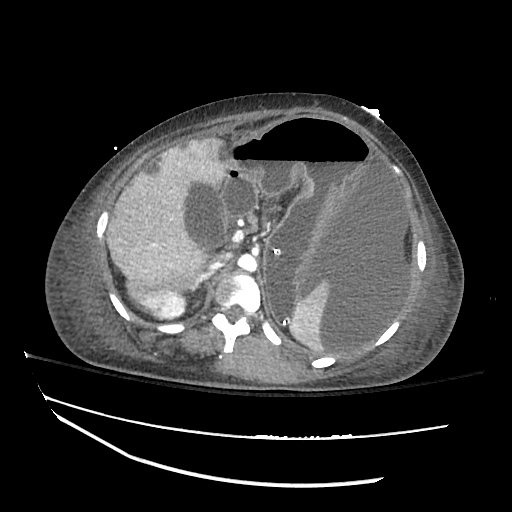
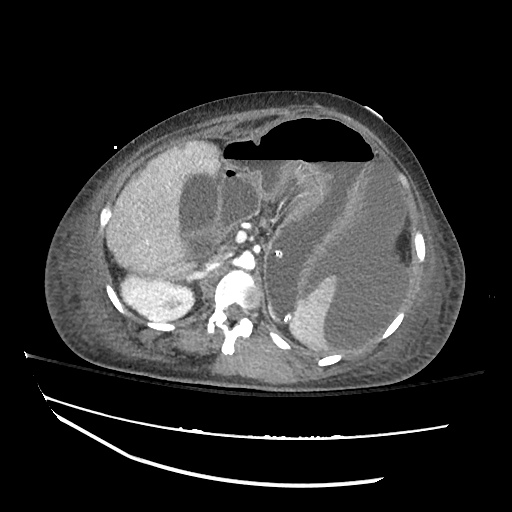
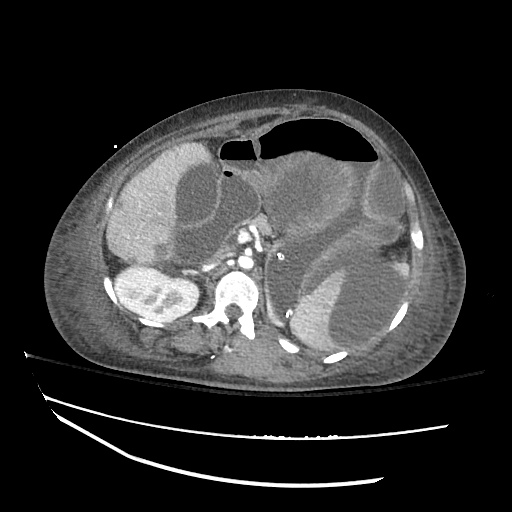
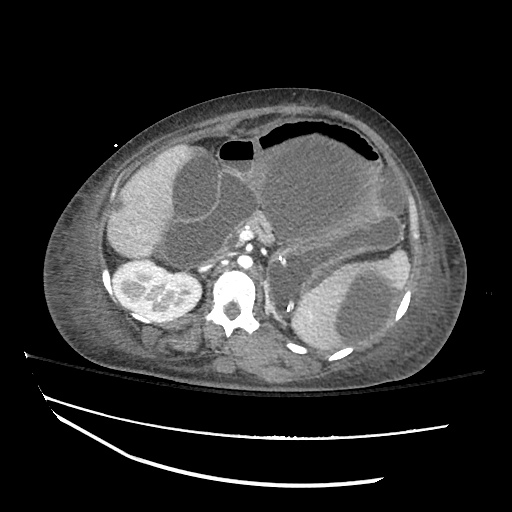
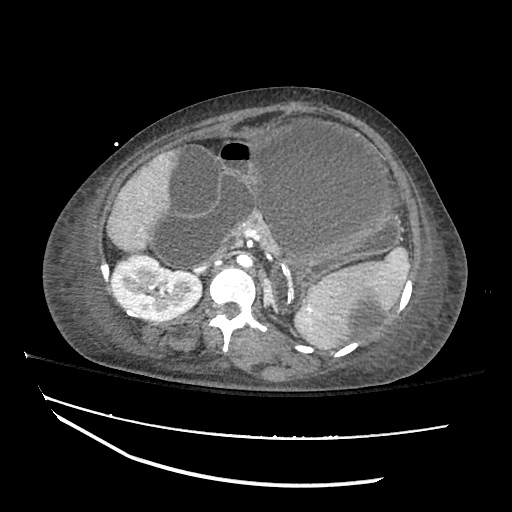
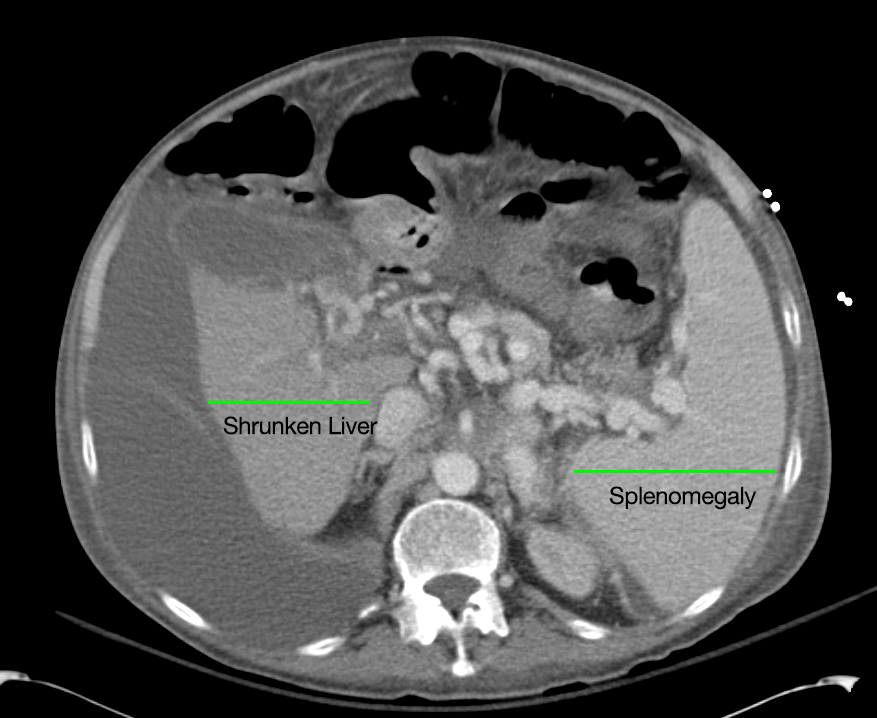
62M with HepC cirrhosis, with e/o decompensation (new-onset ascites) and hyponatremia.
- #Hyponatremia: Sodium 114, likely chronic, patient currently asymptomatic without concerning findings on neurological exam. Clinical findings suggestive of hypervolemic hyponatremia 2/2 decompensated cirrhosis resulting in decreased effective arterial blood volume and volume retention. However, the recent initiation of diuretics, mild AKI and early response to isotonic fluids in the ED suggests possible hypovolemic component.
- 1L fluid restriction
- q.4.h. sodium check, goal increase of 8mEq per 24h
- hold diuretics
- #Hyperkalemia: Potassium 5.6, asymptomatic, AKI vs. medication-induced (aldactone). Continue monitoring.
- #AKI: Elevated creatinine 1.1 from baseline 0.7. Likely pre-renal given recent initiation of diuretics. Consider hepatorenal syndrome given decompensated cirrhosis. Follow-up repeat creatinine after 1L NS bolus in ED.
- #Hepatitis C: decompensated with new-onset ascites. No e/o encephalopathy, continue home rifaximin.
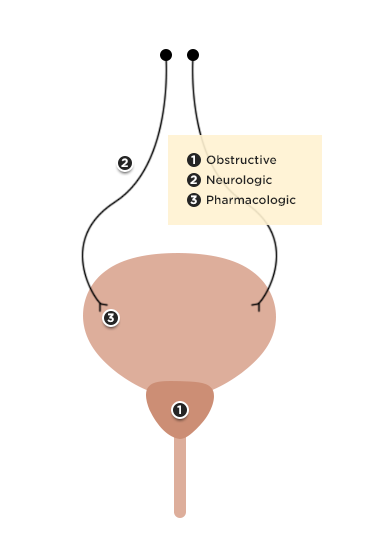
Physiology of Hyponatremia: 1,2,3,4
Differential Diagnosis of Hyponatremia: 5
Evaluation of Hyponatremia: 2
- Identification of onset (acute vs. chronic)
- Presence of symptoms (HA, nausea, confusion, seizures)
- Assessment of volume status (edema, JVD, skin turgor, postural BP)
- Medical history (cardiac, liver, renal disease), drug history
References:
- Freda BJ, Davidson MB, Hall PM. Evaluation of hyponatremia: a little physiology goes a long way. Cleve Clin J Med. 2004;71(8):639–650.
- Biswas M, Davies JS. Hyponatraemia in clinical practice. Postgrad Med J. 2007;83(980):373–378. doi:10.1136/pgmj.2006.056515.
- Adrogué HJ, Madias NE. Hyponatremia. N. Engl. J. Med. 2000;342(21):1581–1589. doi:10.1056/NEJM200005253422107.
- Marx JA, Hockberger RS, Walls RM, Adams JG. Rosen’s emergency medicine: concepts and clinical practice. 2010;1.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056–1062.