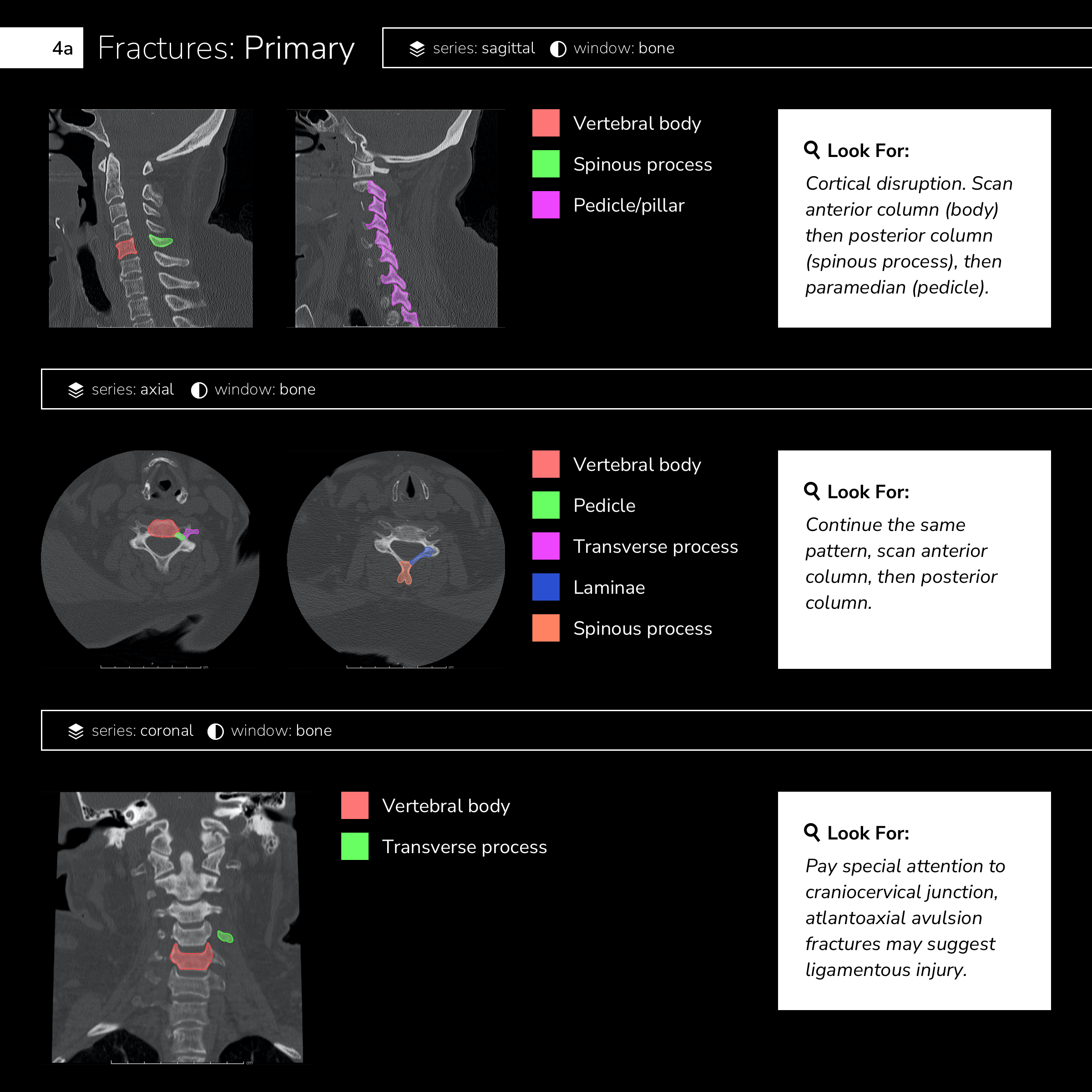
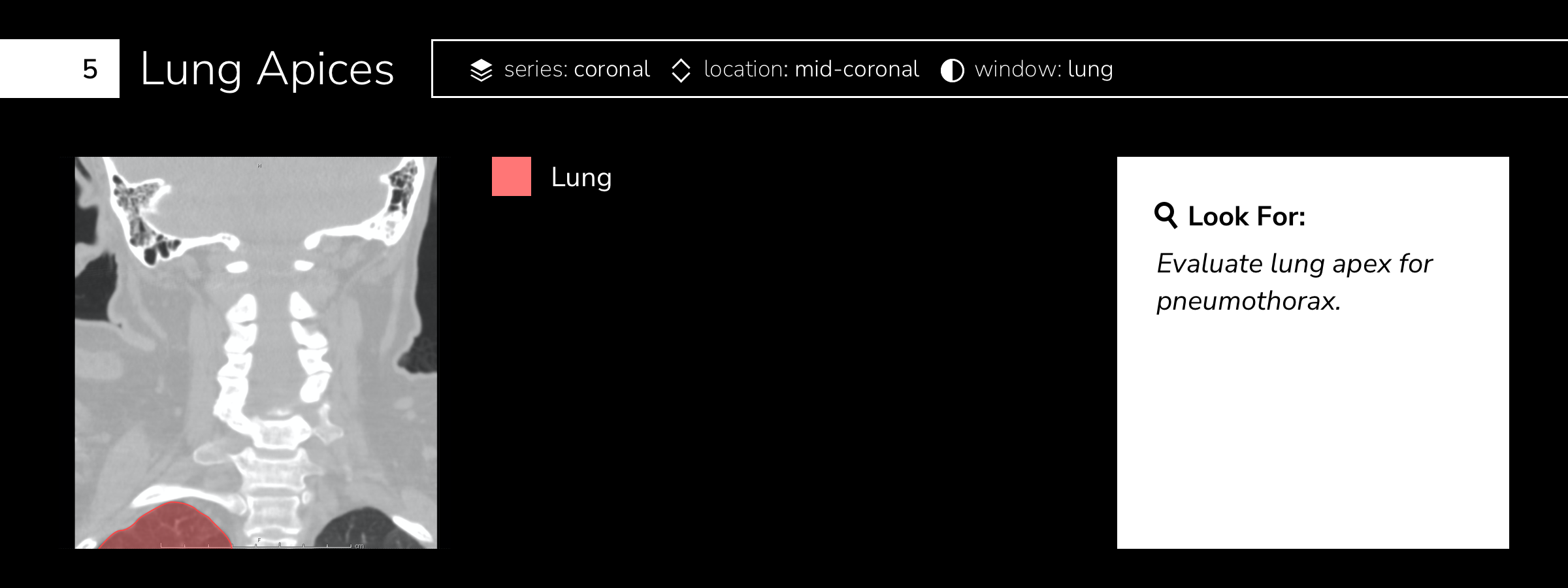
Case 1:
A 20 year-old male with a history of polysubstance use, depression and seasonal allergies presents via ambulance for altered mental status. According to prehospital report, EMS were contacted by the patient’s roommate who noted that he had been acting strangely after being alone in his room for several hours. Vital signs are notable for fever (T 103.2°F) and tachycardia. The patient was confused, unable to follow commands – pupils were dilated.
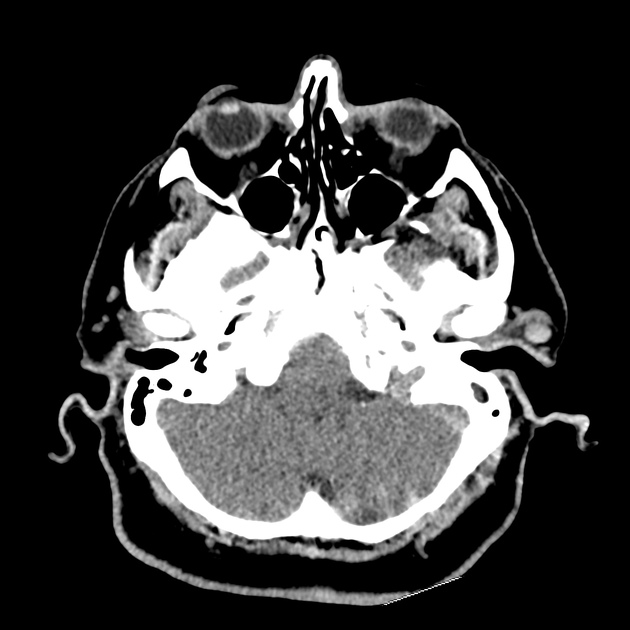
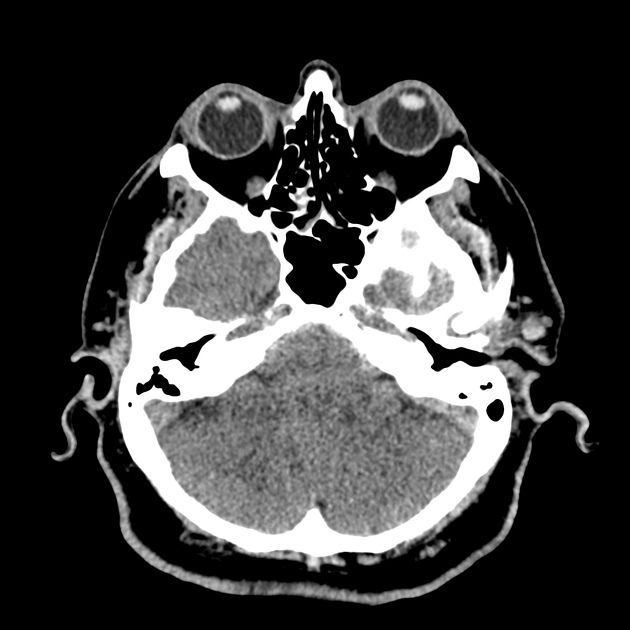
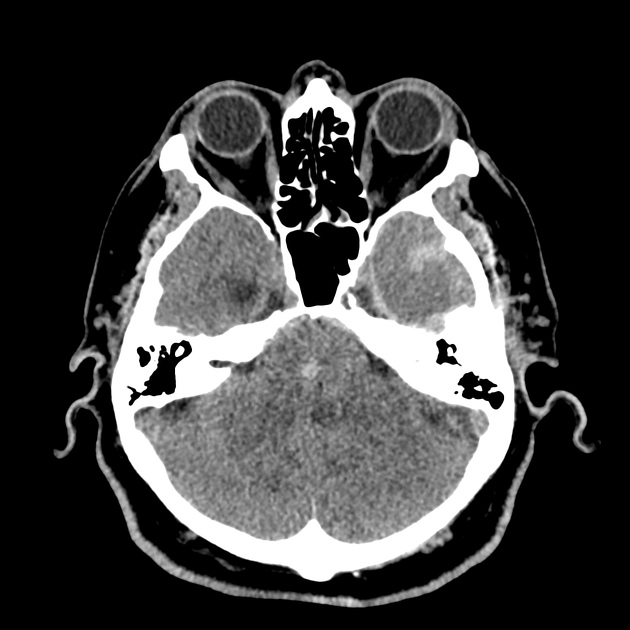
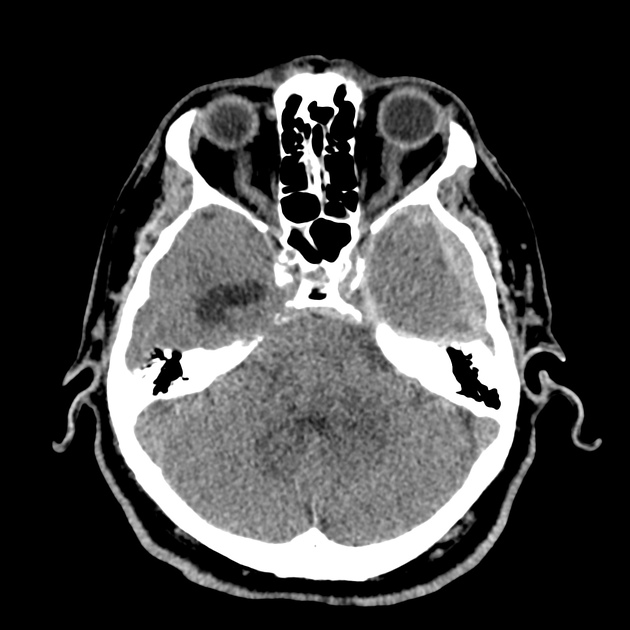
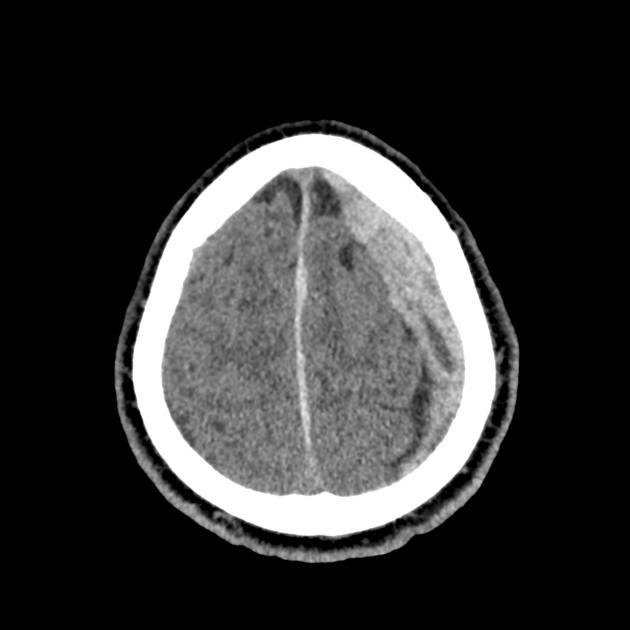
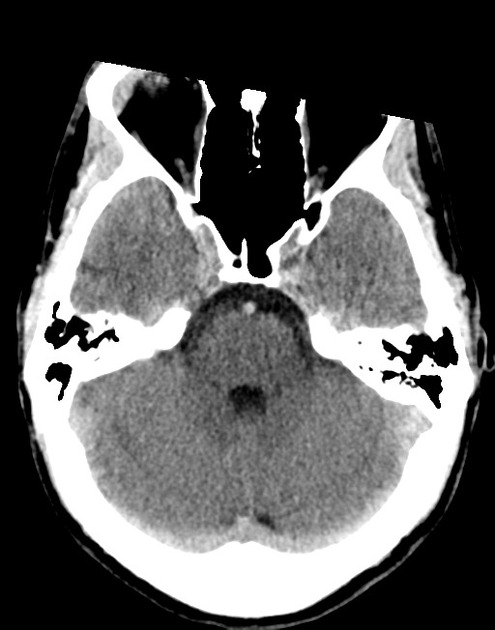
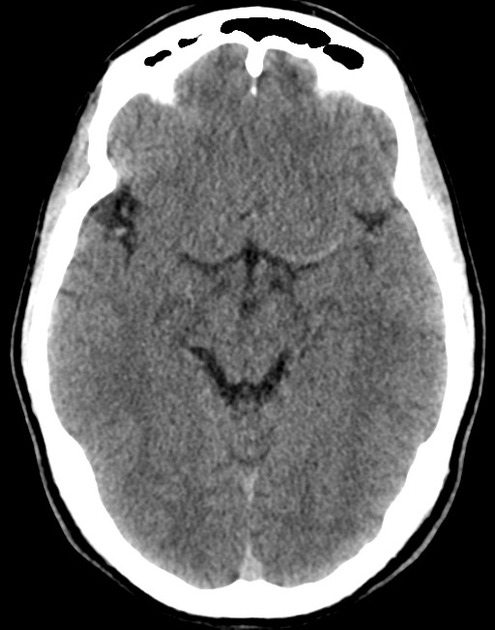
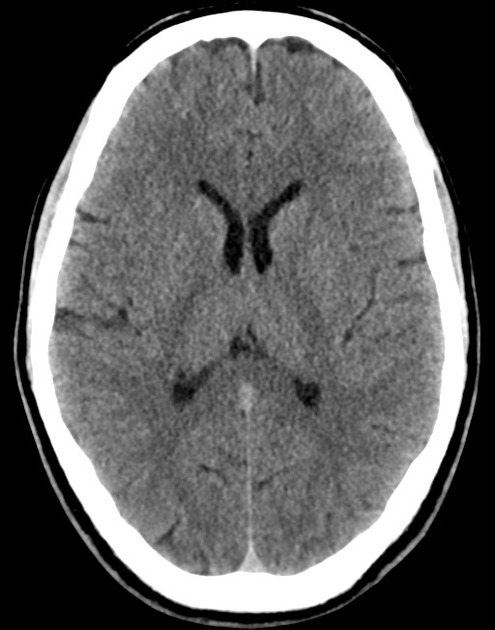
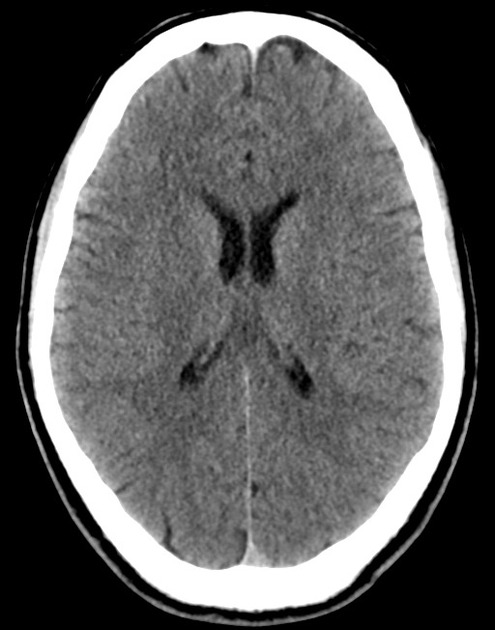
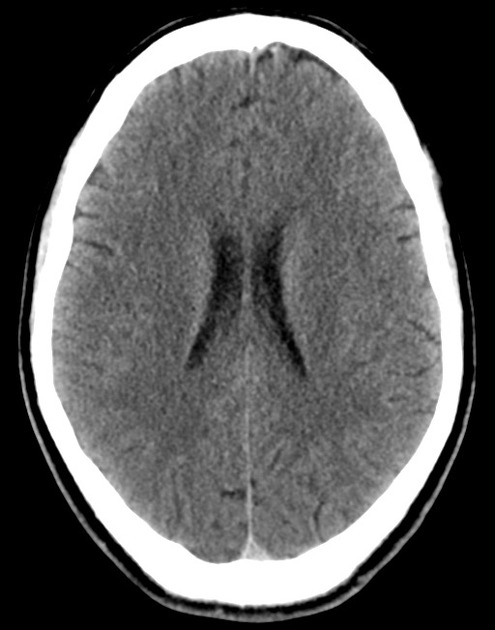
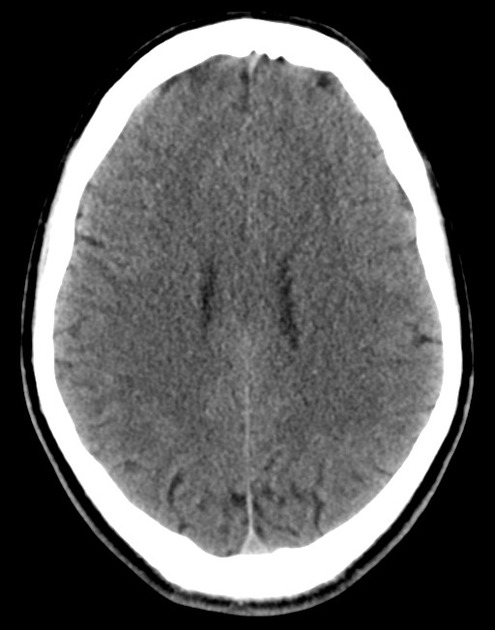
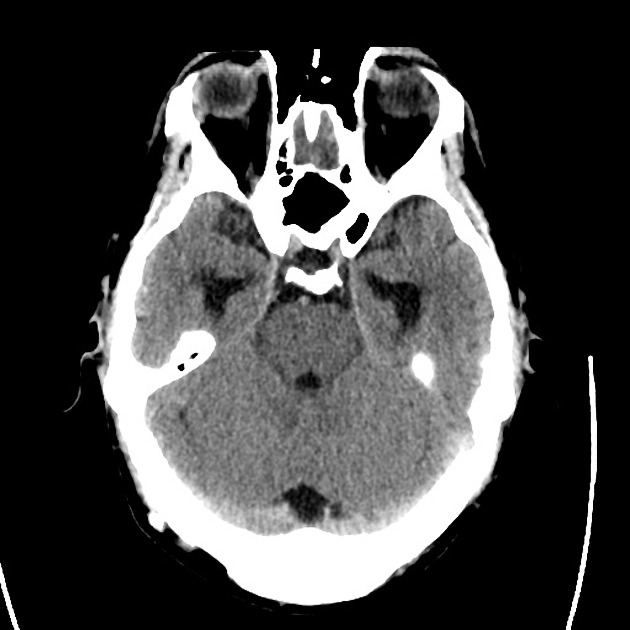
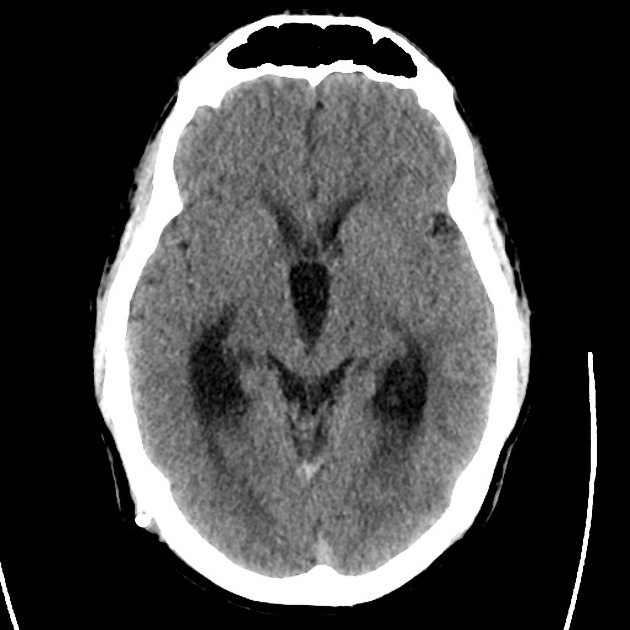
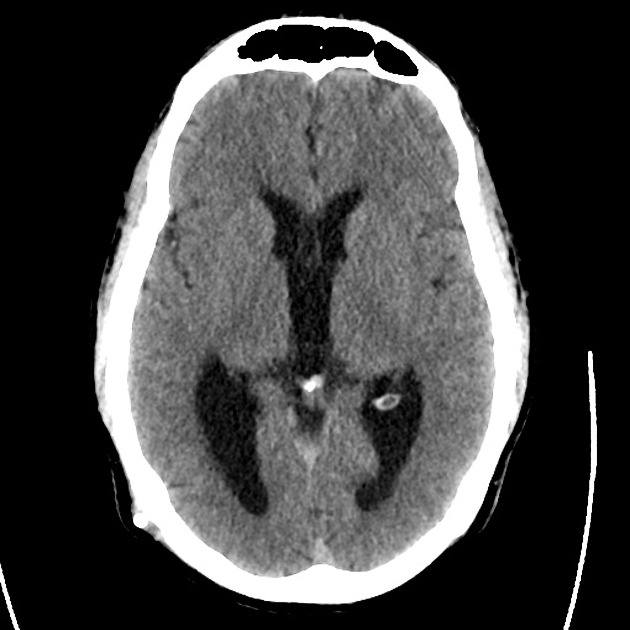
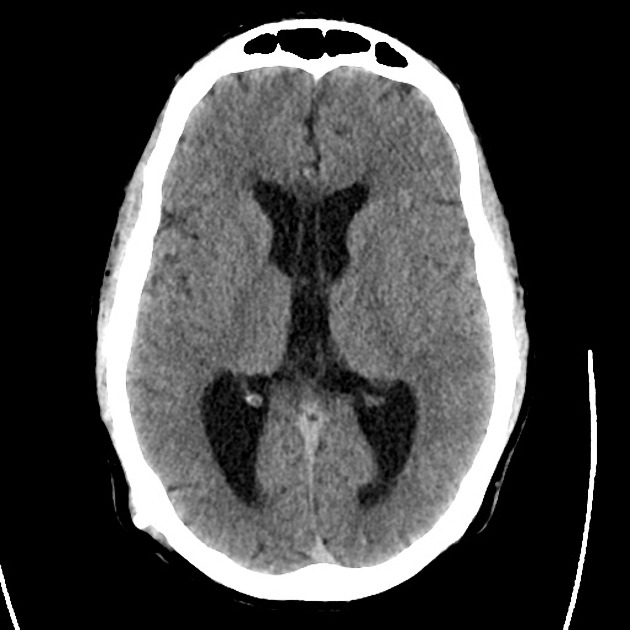
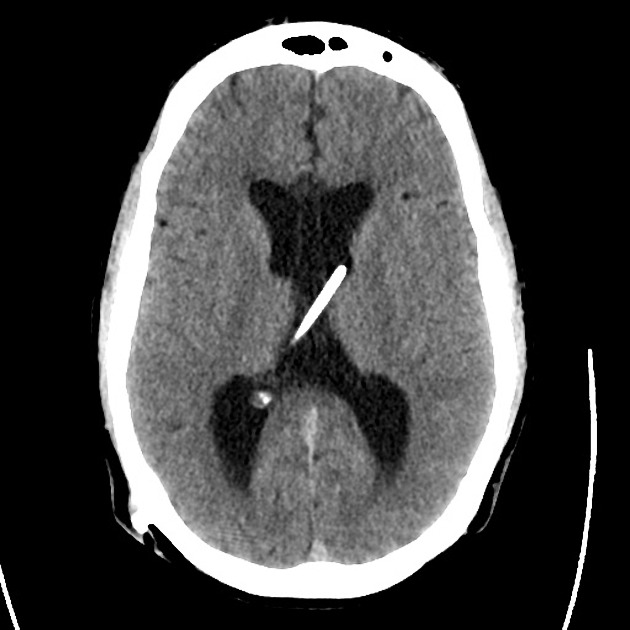
The initial impression was concerning for sympathomimetic toxicity, the patient was treated with cooled intravenous fluids and required pharmacologic sedation and physical restraints to obtain blood samples. ECG, initial laboratory tests and urine toxicology screen were unremarkable. A non-contrast CT head was normal.
The patient remained altered and a repeat examination was performed which revealed multiple, opened blister packs of diphenhydramine and dry, flushed skin.
Anti-cholinergic toxicity was presumed, likely exacerbated by the administration of butyrophenones for sedation. He was treated with benzodiazepines, additional evaporative cooling measures and was admitted to the intensive care unit.
Case 2:
A 32 year-old female with a history of depression was brought to the emergency department by family members who were concerned about bizarre behavior and muscle stiffness. They note that the patient was recently started on a new antidepressant though they are unsure of the name. They describe occasional alcohol consumption but no illicit drug use.
In the emergency department, vital signs were notable for fever and hypertension. Examination demonstrated increased muscle tone and sustained clonus in bilateral lower extremities.
The patient’s presentation was concerning for serotonin syndrome, she was treated with benzodiazepines and intravenous fluids with gradual improvement in mental status and hypertonicity. Upon awakening, she reported doubling her medication dose recently due to persistent feelings of hopelessness as well as increased wine consumption.
An Algorithm for the Evaluation of Toxidromes

Diagnostic Tests
| All Patients |
Most Patients |
Critical Patients |
| POC glucose
Core temperature
ECG
Urine hCG |
BMP
UA
Acetaminophen
Salicylate
Ethanol |
LFT
Lipase
Serum osmolarity
Ionized calcium
Magnesium |
GI Decontamination
Activated charcoal
Activated charcoal (1g/kg) within 1-hour post-ingestion and if the patient is awake and cooperative (or via enteric tube if intubated).
Not recommended
- Heavy metals
- Ions (ex. lithium)
- Corrosives
- Hydrocarbons
- Alcohols
Whole-bowel irrigation
Indicated for sustained-release formulations, expulsion of body packing materials, or ingestion of agent not absorbed by activated charcoal.
Serum alkalinization
For certain ingestions (salicylate, phenobarbital, methotrexate), serum alkalinization through infusion of sodium bicarbonate targeting serum pH 7.5 (and urine pH 8.0) may promote elimination.
Intralipid emulsion
May be useful for local anesthetic toxicity, b-blocker, and calcium channel blocker overdose.
Electrocardiographic Toxidromes
| QT Prolongation |
QRS Prolongation |
| Anti-emetic |
Diphenhydramine |
| Anti-psychotic |
Cocaine |
| Anti-microbials (fluoroquinolone, macrolide) |
Diltiazem, verapamil |
| Anti-depressant (TCA, SSRI) |
Propranolol |
| Anti-arrhythmic |
Amantadine |
|
Carbamazepine |
Gap-producing Toxidromes
Osmolar Gap
- Toxic alcohol
- Ethanol
- Methanol
- Ethylene glycol
- Isopropyl alcohol
- Drug stabilizing agents
- Mannitol
- Propylene glycol
- Glycerol
Anion Gap
- Salicylate
- Iron
- Isoniazid
- Methanol
- Ethylene glycol
- Cyanide
References
- Meehan, T. J. (2018). Approach to the Poisoned Patient. In Rosens emergency medicine: concepts and clinical practice (pp. 1813–1822). Philadelphia, PA: Elsevier.
- Holstege, C., Borek, H. (2012). Toxidromes Critical Care Clinics 28(4), 479-498. https://dx.doi.org/10.1016/j.ccc.2012.07.008
- Mégarbane, B. (2014). Toxidrome-based Approach to Common Poisonings Asia Pacific Journal of Medical Toxicology 3(1), 2-12. https://dx.doi.org/10.22038/apjmt.2014.2463
- Rasimas, J., Sinclair, C. (2017). Assessment and Management of Toxidromes in the Critical Care Unit. Critical care clinics 33(3), 521-541. https://dx.doi.org/10.1016/j.ccc.2017.03.002
- Thompson, T., Theobald, J., Lu, J., Erickson, T. (2014). The general approach to the poisoned patient Disease-a-Month 60(11), 509-524. https://dx.doi.org/10.1016/j.disamonth.2014.10.002
This algorithm was co-developed by Dr. Chigozie Dike, and Dr. Katrina Nemri.
Dr. Dike is a Houstonian true and true, born across the street at Ben Taub Hospital and proud to have the support of her family and friends through her medical school and emergency medicine training at McGovern Med EM at UT Health. She is a big foodie and loves music. In her free time she’s exploring local restaurants, traveling to new cities, and practicing yoga.
Dr. Nemri is currently a second year emergency medicine resident at McGovern Med EM at UT Health. Her interests are in medical education and critical care. She is a local Houstonian who hopes to stay in the city after residency.