The following resource for neonatal resuscitation and neonatal critical care was developed with the guidance of Dr. Agrawal (Neonatology) while on rotation at the White Memorial Medical Center Neonatal Intensive Care Unit.
Endotracheal Tube Size1-3
- Simplified Formula
- Estimated gestational age in weeks ÷ 10 = round to nearest half-size uncuffed tube
NRP Recommendation
| Gestation age (weeks) | Weight (kg) | ETT Size (ID, mm) | Depth (cm from lip) |
|---|---|---|---|
| <28 | <1.0 | 2.5 | 6-7 |
| 28-34 | 1.0-2.0 | 3.0 | 7-8 |
| 34-38 | 2.0-3.0 | 3.5 | 8-9 |
| >38 | >3.0 | 3.5-4.0 | 9-10 |
Laryngoscope Blade Size
| Age | Blade |
|---|---|
| Preterm | 0 |
| Term | 1 |
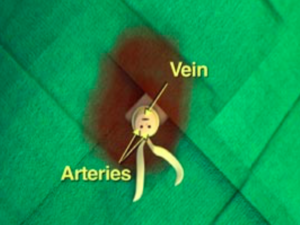
Umbilical Vein Catheter Placement4
- ED Indications
- Unstable neonate
- Contraindications
- Omphalocele
- Gastroschisis
- Necrotizing enterocolitis
- Depth
- 4-5cm or until blood return (for emergent placement)
Umbilical catheter size
| Weight (kg) | Size (F) |
|---|---|
| <1.5 | 3.5 |
| 1.5-3.5 | 5 |
| >3.5 | 8 |
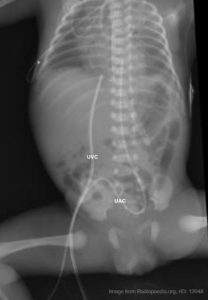
Umbilical catheter positioning on plain radiographs
Umbilical venous catheter position can be verified with a plain radiograph. Positioning within the umbilical vein can be confirmed by tracing a cephalad trajectory from the insertion point at the umbilicus. An umbilical artery catheter will first pass caudally into the internal iliac artery before travelling cephalad into a common iliac artery and the abdominal aorta.
Medications5
| Medication | Dose |
|---|---|
| Epinephrine | 0.1mL/kg (1:10,000) IV, 0.01mg/kg |
| Volume Expansion | 10mL/kg (normal saline, blood) |
| Naloxone | 0.1-0.2mg/kg |
| Dopamine | 5-20mcg/kg/min IV infusion |
Neonatal Physiology and Transition to Extrauterine Life6
An important principle in neonatal resuscitation is supporting the appropriate transition from intra- to extra-uterine life which is dependent on several key anatomic and physiologic changes occurring in an optimal environment.
Anatomy7
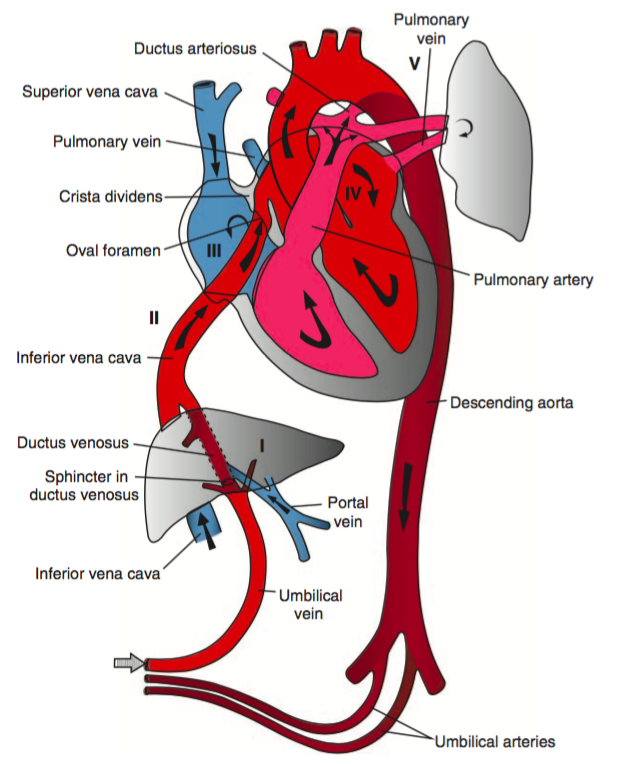
Fetal Circulation
In the fetal circulatory system, oxygenated blood is delivered via the umbilical vein, entering the inferior vena cava via the ductus venosus. The majority of this oxygenated blood passes through the right atrium and into the left atrium through the foramen ovale to enter the systemic circulation.
Meanwhile, high pulmonary pulmonary vascular resistance (due to hypoxic vasoconstriction in fluid-filled alveoli) means that most of the deoxygenated right ventricular output is routed through the ductus arteriosus and enters into the systemic circulation – mixing with oxygenated blood distal to the highest priority end-organs (brain and heart), to be reoxygenated at the placenta.
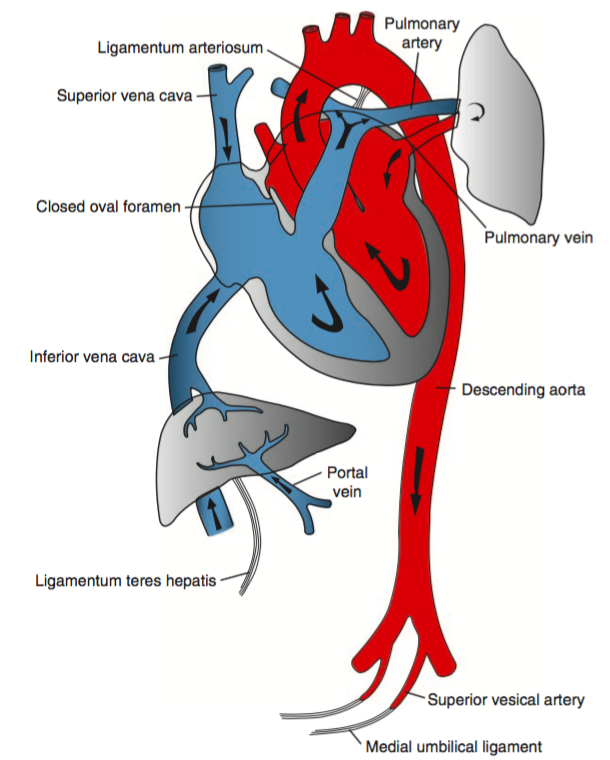
Post-transition Circulation
The transition to extra-uterine life involves several key steps detailed below and is supported by appropriate ventilation, oxygenation and temperature regulation.
- Alveolar Fluid Clearance
Catecholamine and hormone changes (predominantly corticosteroids) during the process of labor induce changes in enzymatic expression that result in the resorption of alveolar fluid into the interstitial space. At the time of delivery, negative intra-thoracic pressure from inspiration further promotes the resorption of alveolar fluid. Mechanical thoracic compression from delivery may also contribute. - Respiration and Breathing
Disconnection from the placenta ceases the transfer of placenta-derived factors including prostaglandins. The withdrawal of tonic inhibition of central respiratory drive from prostaglandins with cord clamping stimulates rhythmic breathing. The infant’s initial breaths and resultant lung expansion promotes alveolar expansion and stimulates surfactant production – this decreases alveolar surface tension, increases lung compliance and further facilitates breathing. - Circulatory Changes
At delivery, clamping the umbilical cord removes a large bed of low-resistance circulation, increasing systemic vascular resistance and systemic blood pressure. At the same time, lung expansion and alveolar aeration decreases pulmonary vascular resistance and pulmonary arterial pressures. At the ductus arteriosus, increased systemic vascular resistance combined with decreased pulmonary vascular resistance decreases shunting and contributes to closure. Similarly, as left atrial pressure approaches and exceeds right atrial pressure, right-to-left flow across the foramen ovale ceases. Collectively, these changes serve to effectively separate the left- and right-sided circulations.
NRP Resuscitation Algorithm5,8
References
- Luten R, Kahn N, Wears R, Kissoon N. Predicting Endotracheal Tube Size by Length in Newborns. J Emerg Med. 2007;32(4):343-347. doi:10.1016/j.jemermed.2007.02.035.
- Peterson J, Johnson N, Deakins K, Wilson-Costello D, Jelovsek JE, Chatburn R. Accuracy of the 7-8-9 Rule for endotracheal tube placement in the neonate. J Perinatol. 2006;26(6):333-336. doi:10.1038/sj.jp.7211503.
- Kempley ST, Moreiras JW, Petrone FL. Endotracheal tube length for neonatal intubation. Resuscitation. 2008;77(3):369-373. doi:10.1016/j.resuscitation.2008.02.002.
- Anderson J, Leonard D, Braner DAV, Lai S, Tegtmeyer K. Videos in Clinical Medicine. Umbilical Vascular Catheterization. Vol 359. 2008:e18. doi:10.1056/NEJMvcm0800666.
- Association AAOPAAH. Textbook of Neonatal Resuscitation. 2016.
- Caraciolo J Fernandes MD. Physiologic transition from intrauterine to extrauterine life. UpToDate.
- Sadler TW. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2011.
- Perlman JM, Wyllie J, Kattwinkel J, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. In: Vol 132. American Heart Association, Inc.; 2015:S204-S241. doi:10.1161/CIR.0000000000000276.


Pingback: Differential Diagnosis of Neonatal Congenital Heart Disease