Brief H&P:
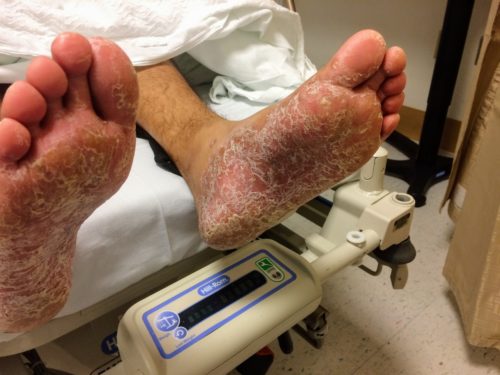
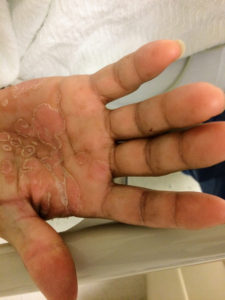
An 8-month old male is brought to the emergency department with fever. He has had four days of fever (temperature ranging from 37-40°C), rash on trunk and extremities, white-colored tongue discoloration, and irritability with decreased oral intake. Temperature on presentation was 39.4°C, examination revealed an erythematous maculopapular rash on the extremities and trunk including soles of the feet. Mucous membrane involvement was noted with oropharyngeal erythema and bilateral conjunctival injection. Neck examination demonstrated right-sided cervical adenopathy.
Labs:
- WBC: 23.4 (N: 59%, B: 21%)
- ESR: 100mm/hr
- CRP: 7.59mg/dL
- Albumin: 3.3g/dL
- AST/ALT: 78U/L, 65U/L
- UA: 7WBC, no bacteria
Hospital Course
The patient was admitted with a diagnosis of Kawasaki Disease and was treated with IVIG and high-dose aspirin. The patient demonstrated marked improvement with treatment and had a normal echocardiogram. He was discharged on hospital day three.
Epidemiology1,2
- Age: 6 months to 5 years
- Northeast Asian
- Possible heritable component
- Seasonal (winter/spring)
Course
- Acute febrile (T > 39°C refractory to anti-pyretics)
- Subacute (coronary vasculitis)
- Convalescent
Diagnosis
- Fever >5d
- Criteria (4/5)
- Conjunctivitis (bilateral, non-exudative)
- Oropharynx changes (strawberry tongue, erythema, perioral)
- Cervical lymphadenopathy (unilateral, >1.5cm)
- Rash
- Extremity changes (erythema, edema, palm/sole involvement)
- Incomplete (2-3 criteria)
Labs
- CBC: Elevated WBC (neutrophil predominant)
- Urinalysis: Sterile pyuria
- Acute phase reactants: Elevated ESR (>40-60mm/hr), CRP (>3.0-3.5mg/dL)
- CMP: Hyponatremia, hypoalbuminemia, hypoproteinemia, elevated transaminases
- ECG: AV block, ischemia/infarction (aneurysm/thrombosis)
- Echocardiography: Decreased LVEF, MR, pericardial effusion
Management
- Hospital admission
- IVIG (2g/kg)
- Aspirin (80mg/kg/day)
Algorithm for the Evaluation of Kawasaki and Incomplete Kawasaki Disease3,4
References:
- Shiari R. Kawasaki Disease; A Review Article. Arch Pediatr Infect Dis. 2014;2(1 SP 154-159).
- Yu JJ. Diagnosis of incomplete Kawasaki disease. Korean J Pediatr. 2012;55(3):83-87. doi:10.3345/kjp.2012.55.3.83.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. doi:10.1542/peds.2004-2182.
- Yellen ES, Gauvreau K, Takahashi M, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125(2):e234-e241. doi:10.1542/peds.2009-0606.